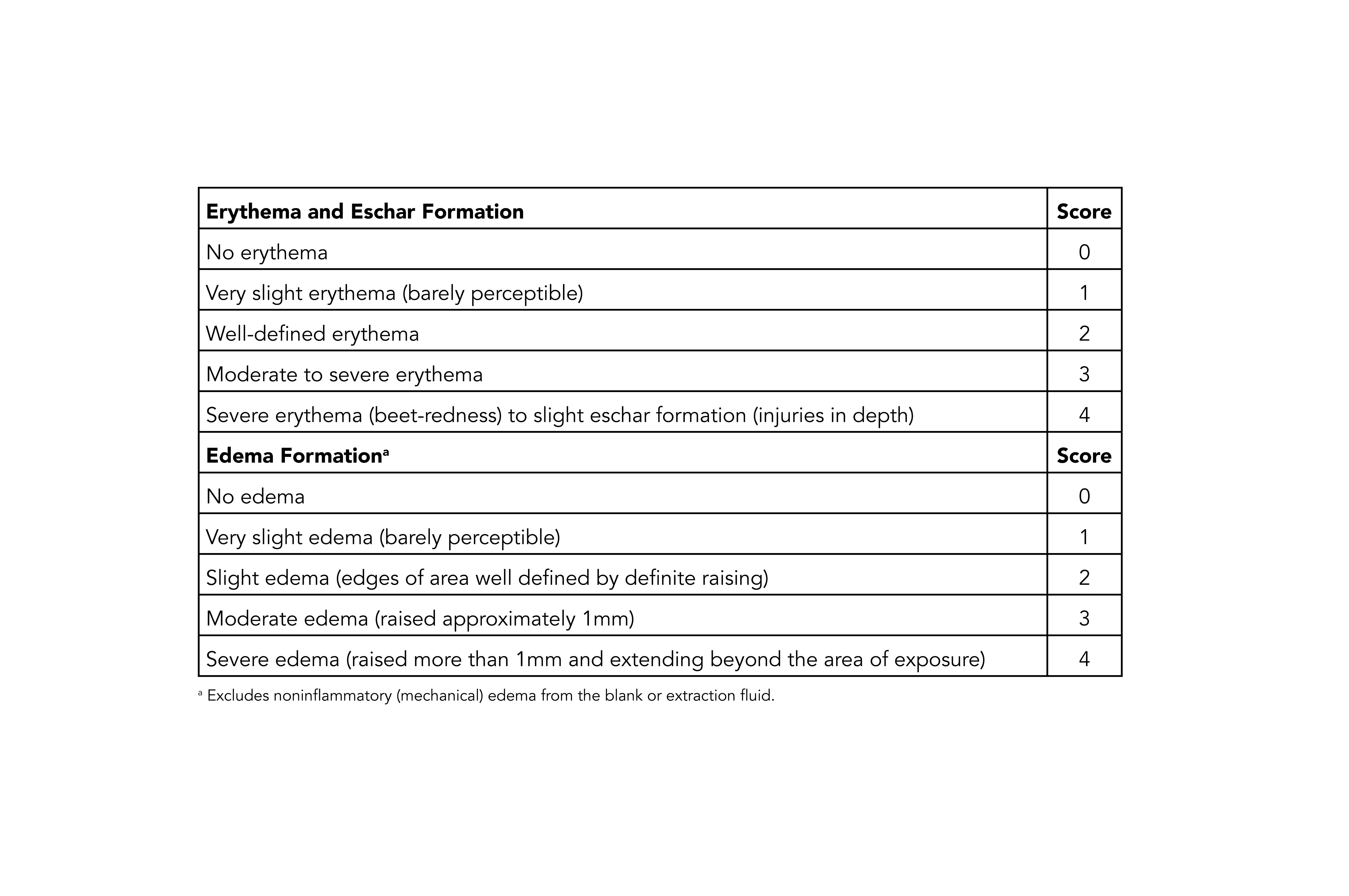
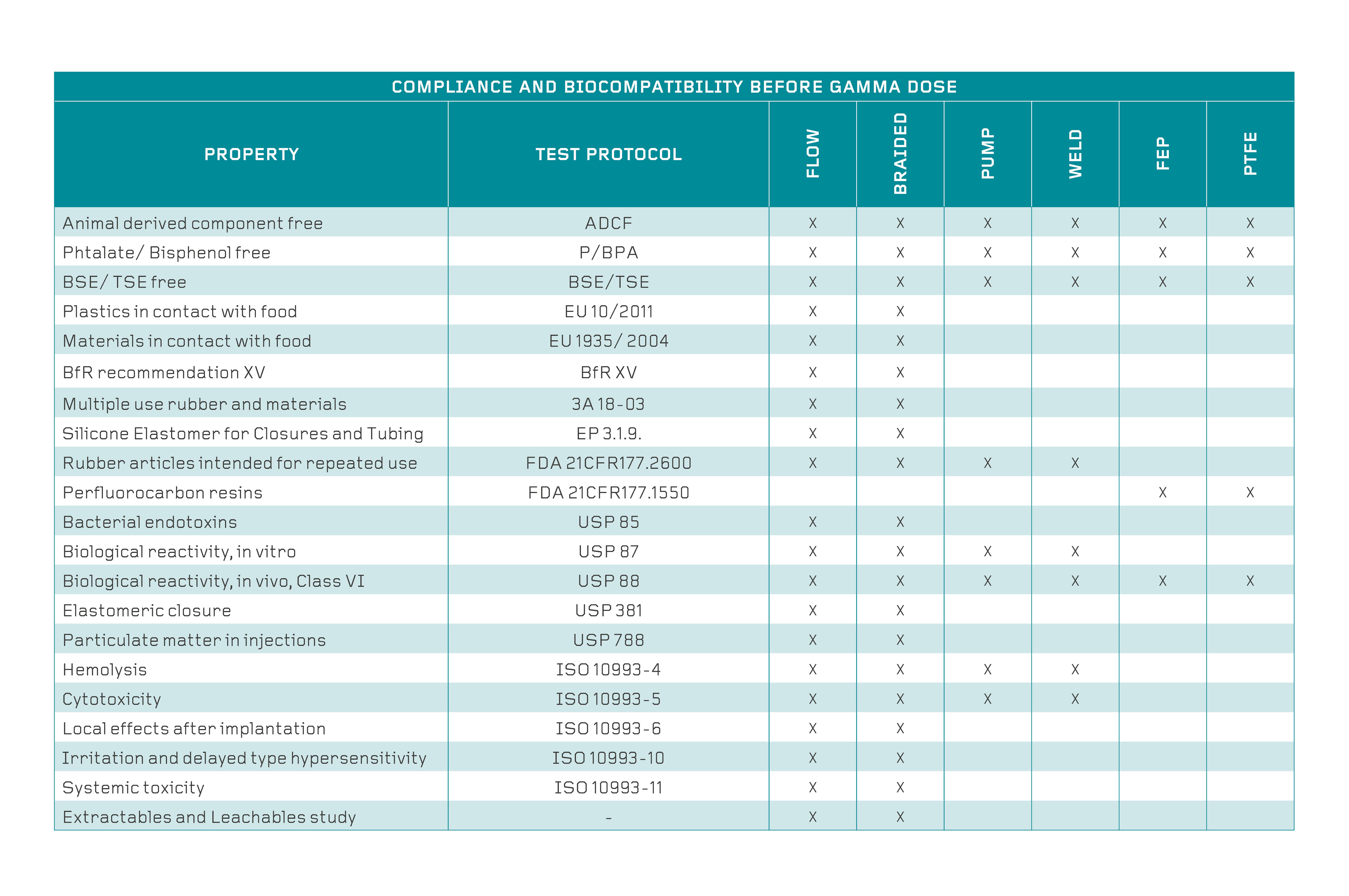
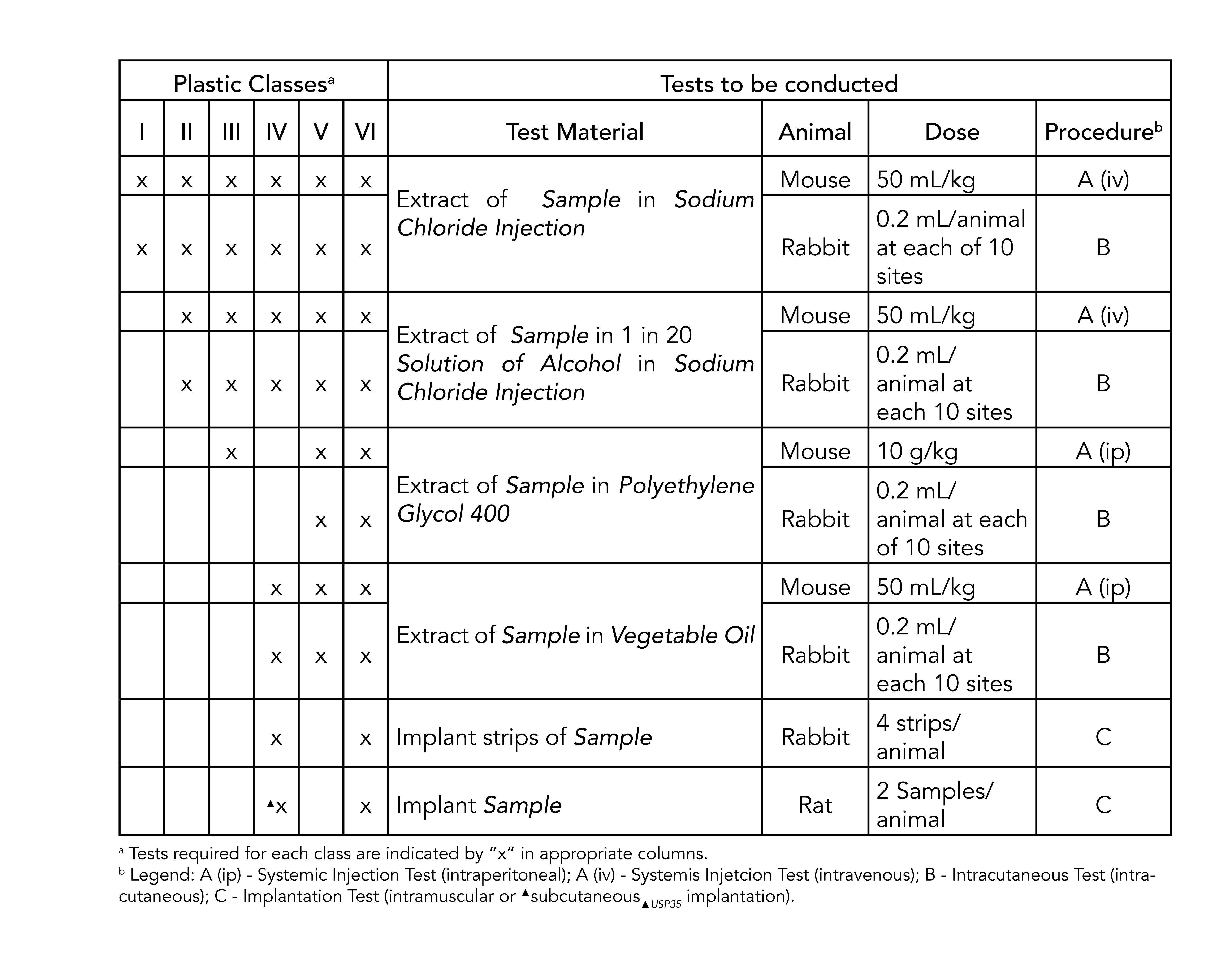
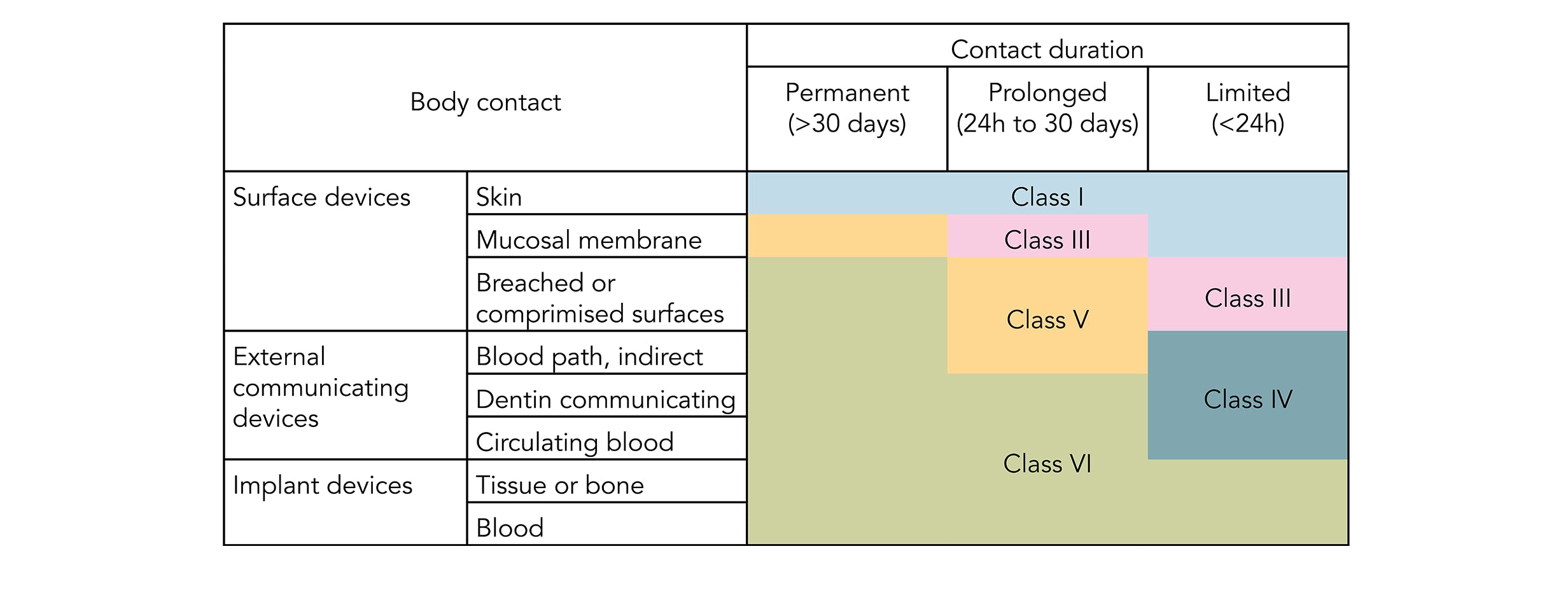
usp class vi elastomers
Sil 714002 USP class VI Silicone 1 70 Yes transl. PPE develops novel elastomer materials to meet the most demanding sealing applications including extreme temperatures and chemically aggressive environments.

Parker Medical Fda O Rings Sealing Devices
038283959 infoeriksbe wwweriksbe ERIKS sa Avenue JE Lenoir 2a 1348 Louvain-La-Neuve tél.

. MIL-STD-810G for fungus resistance. This is the highest grade possible in this test. The Premium Package includes all elastomers in the wetted path of the SLA Series Biotech including O-rings and Valve Seats are USP Class VI and ADI Free and include a full package of certificates.
010483589 infoeriksbe wwweriksbe ERIKS sas. Table 1 shows our standard programme FDA compliant com- FDA and USP class VI compliant. Pharmacopoeia XXII 1190 Class VI Plastics Evaluation.
Specialty Silicone Products SSP makes USP Class VI silicones for medical applications. For this reason the FDA provides a standard 21 CFR1772600 defining allowable rubber compound ingredients and extractibles based on toxicity and carcinogenicity. FAR 25853a for flame retardancy.
Master Bond systems are very versatile and can be used for both disposable and reusable medical devices. Sealable and weldable either pre- or post-sterilization C-Flex 072 provides prolonged pump life Sterilizable by gamma irradiation and autoclave Product Validation Test Summaries available upon request Moldable bondable and formable for single-use assemblies and overmolds Temperature. Pharmacopoeia XXII materials which pass the Class VI Plastic Evaluation are suitable as implantable materials.
A thermoplastic polyurethane elastomer. To keep up with the changes medical device designers and manufacturers need elastomers they can trust. All these special grade products have passed this rigorous test.
Parker recommends its fully traceable USP Class VI compounds for the pharmaceutical industry. Discusses identification testing A workshop Modernization of USP Packaging Standards for Glass and. Moulded O-rings class 1 less than 10 furnace black These can be produced in all possible dimensions up to diameter 1400 mm internal.
Silcon Medicals surface properties resist sticking and encrustation and will not support bacteria growth. Users should confirm by their own tests. 5 hours agoNatron STC 370HP Low coefficient of silicone coating gets USP Cytotoxicity certification.
FDA and USP class VI compliant O-rings High purity elastomers for pharmaceutical biochemical and food industries ERIKS nv Boombekelaan 3 B-2660 Hoboken tel. USP Class VI a a This resin has undergone biocompatibility testing in accordance with US Pharmacopoeia XXII Class VI guidelines Typical Values not to be construed as specifications. Has a full range of specialty adhesives epoxies primers for polyolefins UV curables and silicones that have been fully tested to meet USP Class VI requirements.
It is used in the assembly of medical devices and is capable of withstanding repeated sterilizations including radiation ethylene oxide chemical sterilants and especially autoclaving. In addition this is a great first step towards Class VI medical certification. MIL-STD-883J for thermal stability.
XIAMETER RBL-9200 Series LSR elastomers and XIAMETER RBL-2004 Series LSR elastomers are general-purpose injection-molding materials which are suitable for a wide range of typical silicone rubber applications. Most applications are fairly benign to elastomers. In addition this is a great first step towards Class VI medical certification.
The SSP2390 family of products isnt new but the marketplace for these materials continues to evolve. These products are formulated to meet the requirements of FDA 21 CFR 1772600 and BfR XV. USP Class VI Dow Corning.
These gaskets require no re-torqueing and reduce labor costs. Designates baseline requirements 5. UV10TKMed is USP Class VI approved and meets ISO10993-5 for cytotoxicity requirements.
Expert seal design advice can be provided and components manufactured in the shortest lead times in the sealing industry. USP Class VI for biocompatibility. Ecdel 9966 elastomer meets ISO 10993 andor USP Class VI biocompatibility requirement Ecdel elastomers are plasticizer free copolyester elastomers COPE that offer clarity toughness flexibility and chemical resistance needed in a variety of flexible packaging including flexible medical and pharmaceutical packaging applications.
Inner Dimension - 0375. UL FDA NSF USP Class VI ASTM JIS SAE MIL Specs RoHS Reach. Approved for high pressure fittings and featuring alignment tabs for easy installation.
UL 1203 for explosion-proof. Additionally 22 Material Certifications certifying the composition of all materials in the wetted path and International Calibration Traceability calibrations traceable to NIST or. The transparent elastomer is a high-purity compound with low leachables and extractables as required by the USP Class VI standard for ensuring patient safety.
USP Class VI ISO 10993-5 Cytotoxicity In-Vitro Features Benefi ts. UL 94V-0. Provides a high-level introduction to elastomer chemistry manufacturing technology and the post processing of components 3.
FDA and USP class VI compliant O-rings High purity elastomers for pharmaceutical biochemical and food industries ERIKS nv Boombekelaan 3 B-2660 Hoboken tel. Silcon Medical tubing is made under proper manufacturing procedures and conforms to the biocompatibility requirements of USP Class VI. Sil 714001 USP class VI Silicone 1 70 Yes transl.
The STC 370HP cures to produce a smooth durable frictionless and biocompatible finish. FDA CFR 175300. Tubing USP Class VI Thermoplastic Elastomer meets requirementsfor plastic containers and closures Female MPC and Polycarbonate meets USP Class VI requirementsfor plastics.
Explains basic functional characteristics of components 4. Both ISO 10993 and USP Class VI define testing requirements for biocompatibility the ability of a material to perform a desired function without causing adverse effects on the human body. Like all Natron pad printing inks screen printing inks silicone coatings and elastomers the STC.
As defined in the US. Something that is listed as being USP Class VI demonstrates that the materials utilized are biologically compatible when tested according to the US. For most patient-contact applications a material that meets US Pharmacopeia USP Class VI andor ISO 109933 will be required.
ISO 10993-5 for cytotoxicity.

Usp Class Vi What Is It And How Does It Apply To Elastomers Barnwell

Pharmaceutical And Cosmetics Production
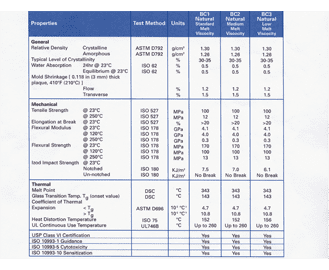
Meaning Of Usp Class Vi Standard United Kingdom

O Rings Fda And Usp Class Vi Darcoid Rubber Company Oakland California
![]()
Usp Vi Silicones Fda Approved Pronat

Iso 10993 Vs Usp Class Vi Medical Molding And Bicompatible Rubber The Rubber Group

Fda Usda Nsf51 Usp Class Vi Compliant Seals Products

Elastomers Todd Technologies Inc

Silicones That Work Specialty Silicone Products Inc

Usp Class Vi What Is It And How Does It Apply To Elastomers Barnwell